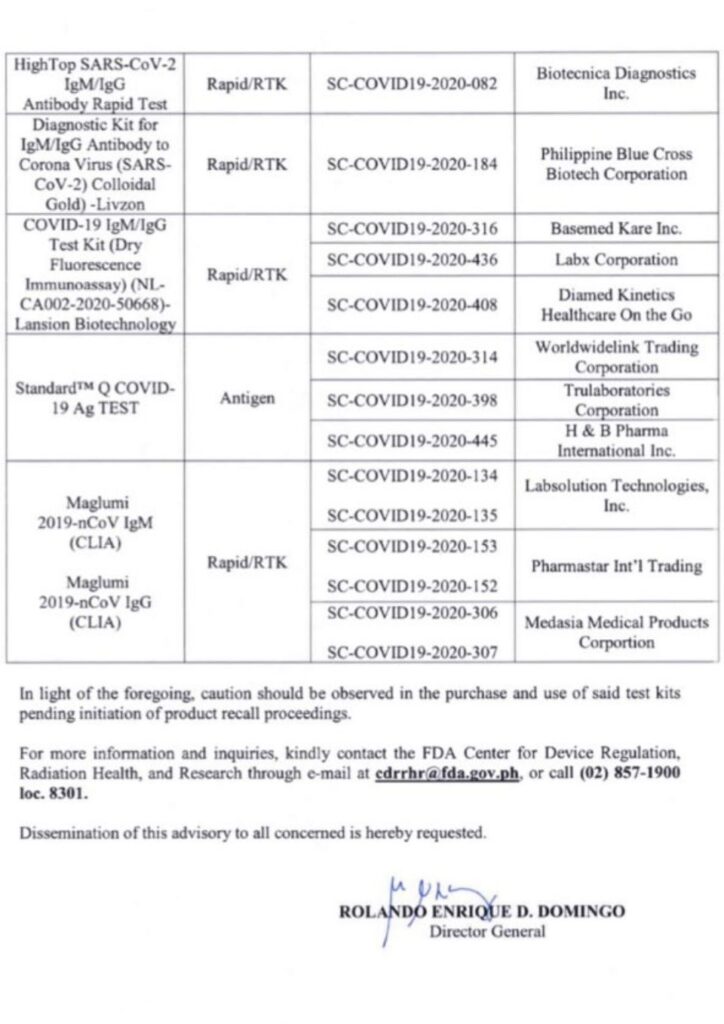
The Food and Drug Administration released a public advisory urging them to be cautious of certain COVID-19 test kits that did not meet their declared product specificity and sensitivity.
Performance validation of such kits are processed by the Research Institute of Tropical Medicine to ensure their accuracy for public use.
The FDA has earlier emphasized that online selling of FDA-certified test kits for COVID-19 are strictly prohibited as they are only intended for medical professional use.
Read the advisory below: